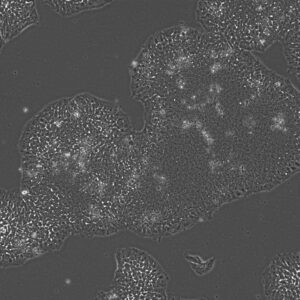
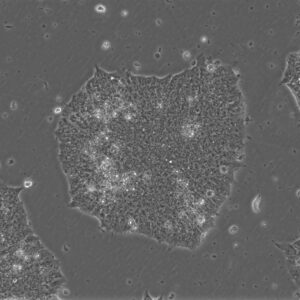
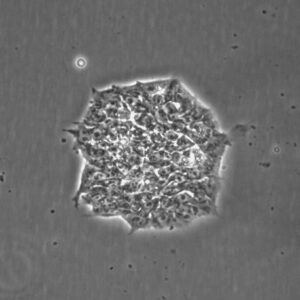
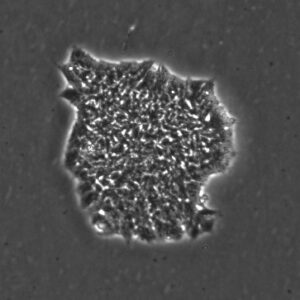
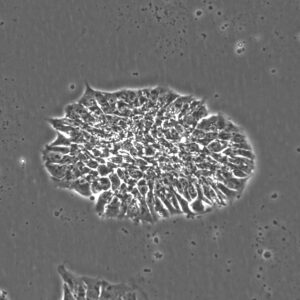
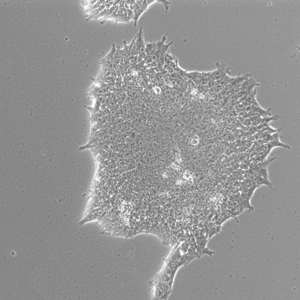
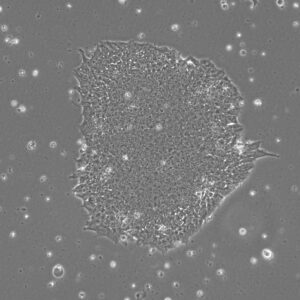
Pluripotent Stem Cells
Pluristyx is proud to offer off-the-shelf NIH-approved Human Pluripotent Stem Cells for immediate evaluation. These iPSC lines are generated from our proprietary cellular reprogramming technology. Pluristyx's catalogue of stem cells are fully characterized to ensure pluripotency and genetic stability of the cells with a clinical application in mind.
iPSCs are generated from regulatory-compliant donors and can be evaluated to assist in the selection of lines appropriate for your research. Our portfolio of cell lines includes both research and GMP clinical grade cell lines. Our products can be used to assist with PSC scale up for clinical product generation.
We offer unmodified lines, gene edited lines and disease-affected versions available for purchase or licensing. Pluristyx is also able to offer custom iPSC lines from clinically compliant material of your choice to match desired donor characteristics.
PluriBank PSCs
Pluristyx PSCs are obtained using IRB approved informed consents and reprogrammed with proprietary mRNA methods. All lines come with Certificates of Testing and have pathways for commercial use. GMP lines are available upon request.
ESI Pluripotent Stem Cells
Pluristyx is a licensed supplier of the ESI brand of pluripotent stem cells (ESI-017, ESI-035, ESI-049, ESI-051, ESI-053). These NIH-registered pluripotent stem cells were derived under Good Tissue Practices (GTP) and are suitable for use in stem cell research and in the development of cellular therapeutics.
Wild-type & disease-affected PSC
Pluristyx is a licensed supplier of wild-type and disease-affected pluripotent stem cell lines from the University of Michigan.
These NIH-registered lines allow means of studying disease progression and treatments.
PluriBank™ iPSCs
Human induced pluripotent stem cells were derived under GTP conditions. Cell lines include wild type as well as gene edited versions of the IPSC products where available. Pluristyx currently provides these cells for research use only.
For use in research applications, contact us for GMP grade cell banks.
ESI PSC
ESI human pluripotent stem cells were derived under GTP conditions making them suitable for use in diagnostic and therapeutic applications. Pluristyx currently provides these cells for research use only.
For use in clinical applications, contact us for GMP grade cell banks.
Pluristyx PSC
Pluripotent stem cell lines that are wild-type and genetically normal. These lines are NIH approved and available for use in R&D.
Contact us for a full list of available cell lines, availability, and lead times.
Pluristyx Disease-Affected PSC
Pluristyx disease-affected human pluripotent stem cells are NIH approved and available for use in R&D. These lines have an identified genetic abnormality that cause disease and enables research into understanding developmental mechanisms of disease establishment and progression.
Contact us for a full list of available cell lines, availability, and lead times.
-
Pluristyx Disease-Affected PSC
UM38-2 PGD Hypertrophic Cardiomyopathy Mutation
Rated 0 out of 5$1,500.00 Select options